Basics of TolerogenixX Technology
Problem
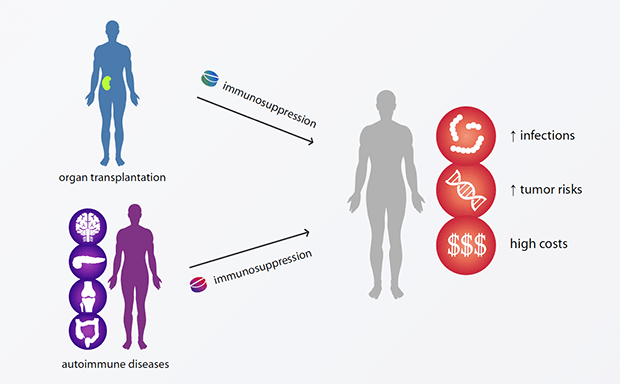
Conventional immunosuppressive medication is associated with a large number of harmful side effects and often results in premature death of patients.
In organ transplantations as well as in autoimmune diseases, the immune system recognizes tissues and organs as non-self and reacts against it.
The current medical answer is standard immunosuppression.
This treatment must be performed throughout and has considerable disadvantages, such as increased infections and tumor risks, as well as high costs.
Solution
The subject of the TolerogenixX method is the specific suppression of the immune reaction in the sense of an individualized (tailored) immunosuppression.
The impact of the TolerogenixX method is increased effectiveness, avoidance of adverse side effects of standard immunosuppression and decisive cost advantage.
Technology
Using the patented treatment of white blood cells, the TolerogenixX method achieves a tailored immunomodulation for the individual patient.
Idea
The idea to develop an alternative to standard immunosuppression came from the desire to no longer treat patients after transplantation and with autoimmune diseases by a broad, untargeted weakening of their body's defenses, but to specifically address the underlying problem.
Conventional drug immunosuppression unfortunately comes with a variety of harmful side effects.
TolerogenixX from Matthias Schaier on Vimeo.
Strengths of the TolerogenixX Method
Specific tailored Therapy
- Specific and effective therapy without side effects
- The simpliest possible approach to individualized cell therapy (ATMP, Advanced Therapy Medicinal Product)
- Platform technology, widely applicable in transplantation and autoimmune diseases
- Novel mode of action, the key tolerogenic cells of the immune system can be directly induced in the patients
- Unwanted reactions of the immune system (rejection, autoimmune reactions, etc.) can be specifically switched off while the desired reactions (defence against pathogens, tumour cells, etc.) are not interrupted.
- Already successfully tested in the clinical phase I TOL-1 study
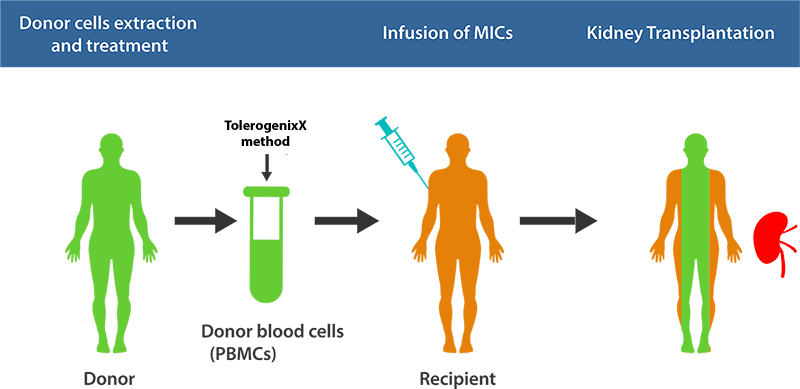
Principle of Application for Transplantation
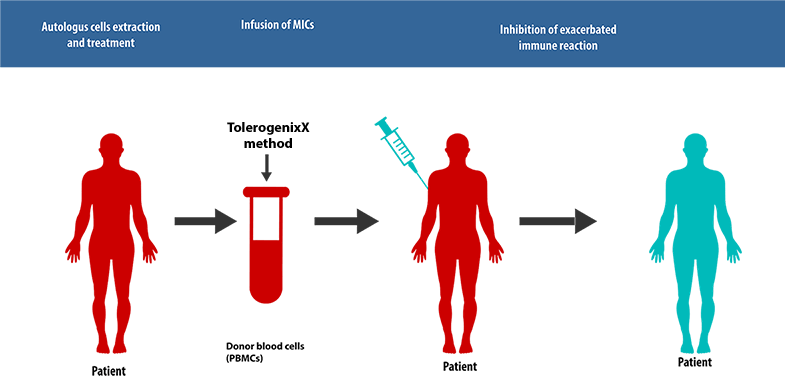
Principle of Application for Autoimmune diseases
The unique feature of the TolerogenixX technology is the safe and reliable suppression of an (unwanted) immune response against a donor organ (transplantation) or self-antigens (autoimmune diseases) without suppression of (wanted) immune responses against third party antigens such as bacteria or viruses.
This tailored immunosuppressive therapy is achieved by the production of tolerogenic MIC cells.
Business
TolerogenixX plans to distribute the MIC cells over 5 hubs worldwide. The first hub is in operation in Heidelberg. The MIC cells can then be used in any hospital throughout the world for the treatment of organ transplant recipients and various autoimmune diseases.
Market
Unlike conventional pharmacotherapy, the TolerogenixX method offers a new therapeutic approach. To date, there are no innovative competitors in this field. In the world, approximately 150,000 patients are submitted to a transplant surgery every year and millions of people suffer from autoimmune disease (e.g. 5 million patients with SLE).
The potential market is worth at least EUR 30 billion. So far, no individualized therapy is available for the different indications.
Competition
The “main competitor” to the TolerogenixX method is the currently used conventional immunosuppression. The disadvantages of this therapy are insufficient prophylaxis of rejection, particularly chronic rejection, and multiple adverse side effects.
Induction of haematopoietic chimaerism through allogenic donor stem cell transplantation following intensive conditioning of the recipient (incl. radiation).
Although these procedures achieve donor-specific immunosuppression or tolerance in some cases, they are associated with extensive side effects, significant logistical hurdles and high costs.
Research and Development
Clinical Application
The clinical application of modified immune cells (MICs) was investigated in a single-center phase-I trial in living-donor kidney transplantation. The prospective organ recipient is transfused with MICs of the organ donor one week prior to transplantation.
Ten patients were treated in within the TOL-1 study. No patients have shown any signs of adverse events. All patients had excellent transplant function. No signs of organ rejection were found in our cohort. All patients are still in clinical follow up.
This is an important milestone that has already been reached: the Phase I study yielded an above-average gain in knowledge. In addition to demonstrating feasibility and safety, clear results on efficacy have already been achieved.
The next Milestone
The next important milestone is a successful Phase II study for the indication kidney transplantation. The TolerogenixX treatment is also very interesting for patients with autoimmune diseases. These can be treated causally for the first time.
TolerogenixX has already published excellent preclinical results in multiple sclerosis (MS) and systemic lupus erythematosus (SLE). Further autoimmune diseases are currently being investigated.